User blogs
3 ESSENTIAL FEATURES OF 'DAILY TRADING RANGE' NEGELECTED BY MOST TRADERS
We
will be discussing a very basic but highly useful resource in today's
lesson which can provide valuable information to the trader.To get more
news about WikiFX, you can visit wikifx news official website.
Essentially,
there are many ways in which you can use a pair's daily trading range
information to help you do better trades. Currency graph is the major
source of reference for traders in order for them to know or identify
the markets conditions other than the economic news. Currency graph is
divided into 2 main axes:
1) X axis: fixed data/information (time)
2) Y axis: fluctuate data/information (currency price)
These
two combinations will produce a wave presenting the change in currency
price is equivalent to the change of time. Currency volatility are
usually move in an approximately similar range according to certain
seasons.
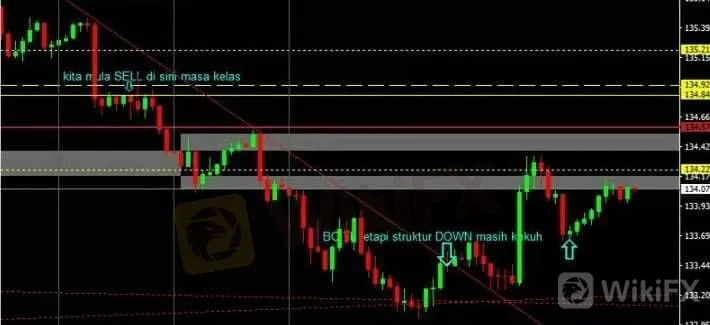
This
range equivalence graph movement can help us to estimate our potential
trade profits or risks based on the entry point within that range
movement.
The range for each currency pair is not identical.
Some of the pairs can move less than 50 pips a day while some of them
can move over 150 pips in a day.
Daily Trading Range is defined as
the average of graph volatility within a day starting from the market is
open until it is closed.
It is measured from the lowest price
to the highest within a day. The way to use DAILY TRADING RANGE is that
you can use the TF Daily, click on candlestick one by one, plot it at
the lowest price and project it to the highest price on the same
candlestick. After that, you will find that the length of the daily
candle is almost similar to the pair.
If you gathered all the
currency pairs and arrange it according to the DAILY TRADING RANGE as
the diagram prepared by me, you will notice that the movement distance
of each currency pair is different. The diagram shown is merely for
illustration as the DAILY TRADING RANGE will change according to the
seasons.
For your information, the DAILY TRADING RANGE will
change over time. It usually changes once a month. So, if you want to
get the DAILY TRADING RANGE for today, you only need to compare the
DAILY TRADING RANGE within a month or 2 months.
Here, you can
compare which of the pairs are move faster and which are move slower. If
you are targeting for a low-risk trading, choose a pair which its DAILY
TRADING RANGE is lower. For the new traders who are just getting
started to trade, it is recommended for you to trade currency pair which
its DAILY TRADING RANGE is lower to minimize the risk of loss.
However,
if you are targeting for a high-risk trading with a rapid and huge
profit potential, you may choose a currency pair which its TRADING RANGE
is higher. Logically, higher trading risk is usually will produce
higher profit potential.
It might be stating the obvious, but your car's headlights are a safety device, and not all headlights are created equal. For a while, carmakers have been fitting powerful LED headlights to their high-end offerings, but more often than not, their cheaper cars—and particularly cheaper trim levels—get saddled with much-weaker illumination. But sometimes a commuter wants to see more of where they're going when the sun goes down. Eventually, they go looking for a solution, starting with their local automotive parts store. But stuffing aftermarket LED headlight bulbs into OEM housings designed for conventional halogen units results in dangerous glare for oncoming drivers. While LEDs can deliver more intense light at a higher end of the spectrum, most aftermarket units also create a hazardous condition.To get more news about car led headlights, you can visit iengniek official website.
The major brick-and-mortar auto parts stores know this, which is why they tend to shy away from aftermarket H11 LED bulbs, other than ones clearly marked for use in fog lamps or "for off-road use only." It's a different world online, with off-brand H11 LED bulb listings on Amazon, eBay, and Walmart websites failing to carry the same prominent warnings.
You can get pulled over for non-spec headlamps, and for a good
reason. In addition to issuing a citation, the law enforcement officer
may have the legal right to force you to remove the bulbs. More
ominously, once the officer has pulled you over, you risk a vehicle
search. With all that in mind, it would be wise to keep a set of
securely packaged OEM bulbs in the glovebox or trunk if you are running
aftermarket LEDs.
With LED technology rapidly evolving, the industry in a constant
beta-test state. While the lighting giants are incredibly cautious in
bringing new products to the market, as drivers we're all guinea pigs on
four wheels for off-brand manufacturers that spin out new bulbs like
chickens lay eggs. The lack of regulation enforcement and objective,
independent testing puts lives at risk.
Although Consumer Reports tests new vehicle headlamps, it hasn't tackled
the topic of LED replacement bulbs, despite Consumer Reports' extensive
resources. A comprehensive Consumer Reports aftermarket LED replacement
bulb test would go a long way to bring clarity to the market. Consumer
Reports' testing of conventional replacement bulbs found that while
aftermarket units can improve headlight brightness, there's much more to
it than that. "Distance and how far a headlight illuminates is governed
more by the reflector (behind the bulb) or the lens (ahead of the
bulbs). While you can change the bulb, you are not changing the
distance, i.e., not necessarily improving safety."
High-end headlights are now a must if an automaker wants the coveted Insurance Institute for Highway Safety (IIHS) Top Safety Pick+ designation for a particular model of car. While IIHS performs exhaustive headlamp testing with new cars and SUVs in their laboratory and test track, it does not have an official position on aftermarket replacement bulbs for older vehicles. The IIHS recommends that you make your vehicle purchase decisions carefully, considering not just the make and model but the specific trim level.
Automakers have embraced light emitting diodes (LEDs) in headlights, and their use is becoming more widespread. At Consumer Reports, 55 percent of the 2018 models we tested had LED headlights. Of the 2019 models we've tested, 86 percent had LEDs.To get more news about led headlights, you can visit iengniek official website.
LEDs are small and can be used in a string of lights, giving car designers more leeway in how the headlights look. But in CR's testing, we discovered that these new lights don’t offer any more illumination than traditional halogen and/or high intensity discharge (HID) headlights.
The problem for many consumers is that they’re paying more for the LEDs but not getting much bang—if any—for that extra buck, says Jennifer Stockburger, director of operations at the Consumer Reports Auto Test Center.
“Yes, they’re stylish, but drivers need lights that will make them safer, and not just make a fashion statement,” Stockburger says. “Car shoppers need to think about headlights as a safety feature in the same way they think about brakes or even seatbelts.” All headlights illuminate the road ahead in one of two ways, Stockburger says. The first, traditional way is to use a reflector to bounce the light from the bulb forward. The other way is to use what is known as a projector, where the headlights use a lens that focuses and directs the light outward.
There are several ways for headlights to create illumination. Halogen bulbs heat a filament to the point where it emits light. They’re the most common on U.S. roads, and they typically give off a yellowish tint.
HID headlights are less common, and they work by igniting a gas—most often Xenon—with electricity inside a bulb. They emit a white or bluish-white hue.LEDs are a far more advanced technology. There are two semiconductors (on a small chip) with either a surplus or a small number of electrons. When the two semiconductors have an electrical charge applied to them, atoms move toward each other and combine, and the resulting energy that's created is released as light.
LED headlights first appeared in the U.S. on the Lexus LS 600h sedan back in 2007, and they were originally found only on high-end cars. But LEDs began appearing in more mainstream models about eight years ago, and some of them impressed us in our testing.
The 2015 Cadillac Escalade’s LEDs, for example, were the best-performing headlights we had tested up to that point. Phil Leinert, a communications manager at General Motors, notes that the Escalade was the first SUV sold in the U.S. with all-LED headlights.
The demand for space on World of Warcraft Classic servers, which launched tonight at 6 p.m. Eastern Time, has been overwhelming.To get more news about Shadowlands WoW Gold, you can visit lootwowgold news official website.
Fans of the nearly 15-year-old game have been creating characters like mad since last week, reserving their names and a shot at recapturing the nostalgia of playing the game as it was close to its launch. There is no up-front cost to Classic; if you have an active World of Warcraft subscription, you own it. So the barrier to those wanting to take a gander at the game, whether they stay or not, is very low.
As a result, nearly every server at launch was reading Full before the game even began, and Blizzard's been posting increasingly plaintive notes asking people to move off ultra-full servers where streamers or popular communities have taken up residence to newer, lower-population servers.
Realistically, WoW game director Ion Hazzikostas told Forbes, the population is going to drop dramatically after the tourists leave and the committed WoW Classic players settle in for the long haul. To deal with that disparity, the company is using "layering," which creates multiple copies of the entire game world of Azeroth to hold people in the short term.
I caught up with Hazzikostas for an extended interview about those
realm login queues, the reasons for layering, and how it works. This is
the first part of that interview, talking specifically about why you're
probably waiting in a login queue right about now. (Welcome! Can we get
you a cup of coffee and a few good stories to read while you wait?)
To be clear, we don't view layering as an improvement to the Classic
experience. We're not saying that man, we wish we had layering back in
2006, it would have made it so much better, but now we have it, so let's
do this. Layering is the lesser of several evils by a large margin.
We are trying to manage long term healthy populations on these servers
around a unique sort of game launch. There's no box that you have to buy
on a shelf in a retail store for an outlay of dollars. We are opening
this world up to millions of people, many of whom are just going to want
to check it out as a matter of curiosity.
There are others who've been waiting for this clearly for years and they are in, as in as can be, but they're all going to be there contending for the same server space on day one.
Say goodbye to unrewarding PvP kills. The PvP Honor System will be implemented on November 12! Get ready to rack up those kills because things are heating up. Arm yourself with the best gear, or start farming WoW Classic gold for them. You don’t want to get caught flatfooted at the starting line of the race to get ranks, do you?To get more news about WoW Gold Classic, you can visit lootwowgold news official website.
It is a way to keep track of the PvP kills of a player. Accumulating ‘honorable kills’ (HK) will increase your contribution points (CP). Those points will determine your ranking points. Along with your HK, ranking points influence your final rank. Ranking only measures your contributions to PvP and is not a level of anything. It’s a measure of how active you are.
Now, HK isn’t the only way to farm contribution points. Completing Battleground objectives in later phases will also give you contribution points. It’s a better way to farm points. When you kill the same person multiple times within 24 hours, you get diminishing returns. That’s why, even when participating in long Battleground games and having over 1000 HK, you get less CP than when completing objectives. You can circumvent this by joining many short games against a different set of players each time.
Party members will get the same amount of CP as the host. There is less gain or none at all if the target is too weak or is too low ranking compared to you. Conversely, if you defeat equal to you or higher-ranked players, you get more points.
Rankings are calculated once per week. If you don’t participate, your
rank will decay. Since it’s a measure of your activity, you only keep
your rank if you do the bare minimum. To increase your rank, you have to
be more and more active in world PvP and Battlegrounds.
Yes, the system brings rewards to those who participate. Aside from
bragging rights and the feeling of superiority, you can get sweet armor
based on your rank. Lower ranks get accessories and trinkets. Higher
ranks get armor of superior quality and above. There’s even a mount
available to those who get at least rank 11. As early as rank 2, you get
an insignia that can remove various debuffs from your character.
If you’re worried that you’ll drop in rank and lose those rewards, don’t be. Future patches let players keep their rewards when they do. It’s just a matter of time before World of Warcraft Classic catches up.
China's phone industry urged to 'get back to work'
Analysts predict smartphone shipments within China will drop by around 40% in the first quarter as the coronavirus disrupts the country's supply chain.To get more news about china industry research, you can visit acem.sjtu.edu.cn official website.
Numerous factories across China have suspended production as the country continues to cope with the outbreak.
Lei Jun, who is from the Hubei province where the virus originated, said he was concerned for his home region.
"I'm from Hubei and spent four years in Wuhan in college, so my feelings for Wuhan are quite deep," said Lei, while donning a facemask at the launch of the company's new flagship Mi 10 phone series.
Xiaomi was the world's fourth-biggest smartphone maker in 2018, according to market research firm IDC - after Samsung, Apple and Huawei - and opened a second headquarters in Wuhan in December.
He added: "I believe Wuhan is a glorious city, and I believe even more that the brave and optimistic people of Wuhan can definitely fight this virus."The economic fallout from the outbreak of the virus, named Covid-19, is spreading far and wide, moving across China's manufacturing sector, major airlines and global supply chains.
Although Chinese workers were due back at work this week after an extended New Year holiday, many factories and offices have remained closed.
Google temporarily closed its offices in China, Hong Kong and Taiwan amid the outbreak, and other tech giants, including Amazon and Microsoft, quickly followed suit.
It has led to some analysts estimating that global smartphone shipments could fall by as much as 10% this year, and cause a shortage of iPhones, and the new iPhone 11 in particular.
China's President, Xi Jinping, was reported on Monday by media in the Far East to have pledged to prevent large-scale layoffs of workers in the wake of the virus's continuing economic impact.
This study focuses on China's Elevators industry assessments and company profiles. In the two past decades, the industry has been growing at a fast pace. The dramatic expansions of the manufacturing capabilities and rising consumer consumptions in China have transformed China's society and economy. China is one of the world's major producers for industrial and consumer products. Far outpacing other economies in the world, China is the world's fastest growing market for the consumption of goods and services. The Chinese economy maintains a high speed growth which has been stimulated by the consecutive increases of industrial output, imports & exports, consumer consumption and capital investment for over two decades. Rapid consolidation between medium and large players is anticipated since the Chinese government has been encouraging industry consolidation with an effort to regulate the industry and to improve competitiveness in the world market.To get more news about chinese industry and management practice, you can visit acem.sjtu.edu.cn official website.
Although China has enjoyed the benefits of an expanding market for production and distribution, the industry is suffering from minimal innovation and investment in R&D and new product development. The sector's economies of scale have yet to be achieved. Most domestic manufacturers lack the autonomic intellectual property and financial resources to develop their own brand name products.
This new study analyzes the industry structure, capacities and output. Major producers' production locations, market shares and profiles are presented. The primary and secondary research was done in China in order to access up-to-date government regulations, market information and industry data. Data was collected from Chinese government publications, Chinese language newspapers and magazines, industry associations, local governments' industry bureaus, industry publications, and in-house databases.
This year marks the 20th anniversary of MBA education in China,” says Ma Jia, director of marketing and admissions for Tsinghua University’s IMBA program, adding that the speed at which the market has grown is, like most things in China, astounding. “China grew fast from the original nine programs to 236 programs.”To get more news about top mba colleges in China, you can visit acem.sjtu.edu.cn official website.
Twenty years on, it is unlikely such expansion will continue for much longer. Instead, Chinese business schools are competing now on the quality of their education.
Competition among China’s business schools is set to become much more fierce in the coming years as students - both international and Chinese - begin to recognize the value of gaining their MBA in China instead of traditional centers such as Western Europe and the US.
At present there are a few well-recognized business schools offering international-standard MBAs in China and yet more offering MBAs partnered with Western schools such as the Sloan School of Management at the Massachusetts Institute of Technology (MIT: Sloan), which partners with Tsinghua University’s School of Economics and Management, amongst others.
With such variety in China though, choosing the right MBA is crucial.
There are nevertheless some distinct benefits and drawbacks that apply to MBA programs in China regardless of the individual business school.
Perhaps the most common benefit of studying for an MBA in China is the widely accepted view that, as a developing marketplace, MBA holders see their career development progress much faster than in more mature economies.
The rapid movement of industry to China requires a great deal of well-qualified managers who can understand both Western and Chinese business practices. Moreover, a benefit to students considering studying for an MBA in China is the current lack of such talent throughout the country.
“The lack of management talent is quite significant,” says Ms Ma. “MBA graduates will have more job opportunities and room for development.”Obtaining an MBA in China allows students to see firsthand what business practices in China look like, and will give those hoping to work in China a significant head start over those planning on transferring their MBA from the West to China.
Transferable experience does not only concern moving from West to East but also concerns moving within China itself, as the distinction between Hong Kong and China must be made clear.
At times Hong Kong, a Special Administrative Region (SAR) of China, is indistinguishable from the People’s Republic, but in terms of MBA education there are some acute differences in the experience an MBA student can gain.
"Hong Kong has a lot of competitive MBA courses with highly diversified student demographics," says Patrick Groom, international admissions manager at Cheung Kong Graduate School of Business in Beijing. "Often, Hong Kong students will be the minority in an MBA course, such is the multi-national make up of Hong Kong MBA students.”
But the courses available in Hong Kong, he says, look West a great deal more than they really examine business operations in China and China's impact on the rest of the world.
"Students that study in Hong Kong can lack a genuine foothold in mainland China's business environment as well as an understanding of the mainland's cultural differences, when compared with those who take their MBA in the PRC," he adds.
China's economy, as shown by multiple mid-year indicators, has ridden out its downturn due to COVID-19 strains and bounced back to growth in the second quarter (Q2). Economists believe that the country's V-shaped recovery is only getting started.To get more China economy news, you can visit shine news official website.
In Q2, China's gross domestic product expanded by 3.2 percent year on year, reversing a 6.8-percent contraction in the previous quarter. China's fiscal revenue marked the first expansion this year by gaining 3.2 percent year on year in June, while the contraction of the retail sector declined markedly.China's economy has gradually emerged from the slump and returned to the level it was roughly at prior to the outbreak, backed by the stimulation that has delivered burgeoning signs of work resumption, industrial chains and services sector," said Shao Yu, chief economist at Orient Securities.
Latest data showed that the purchasing managers' index (PMI) for China's manufacturing sector rose to 51.1 in July from 50.9 in June, remaining in expansion territory for the fifth month in a row, indicating stronger confidence of market entities.
"The steadily firming recovery points to the effectiveness of China's epidemic prevention and pro-growth policies to boost production and domestic consumption," said Sheng Hai, a macro analyst with China Industrial Securities.
His point was echoed by Steven Zhang, chief economist at Morgan Stanley Huaxin Securities. "China has its institutional advantages that enable a more agile and rapid response to public safety emergencies like COVID-19."The prompt introduction and implementation of an array of measures, including higher fiscal spending, tax relief and cuts in lending rates and banks' reserve requirements to revive the economy and support employment, according to Zhang, is one of the major reasons behind the Q2 positive growth.
As recent data showed that China's imports from emerging countries increased significantly, the recovery of the world's second-largest economy is expected to boost the pace of other economies' restoration, according to Zhang.
In H1, China's trade with ASEAN went up 5.6 percent year on year to 2.09 trillion yuan (about 301.12 billion U.S. dollars), while that with countries along the Belt and Road (B&R) accounted for 29.5 percent of the total trade, up 0.7 percentage points year on year.
Zhang said that trade between China and other parts of Asia, B&R countries and ASEAN is expected to further expand as the spillover effects will be more notable due to shorter distance and lower logistics costs.To mitigate the impact of the COVID-19 outbreak, China has been investing heavily in infrastructure projects through local government bond issuance, which is expected to buoy demand for bulk commodities in the global market, thereby benefiting B&R countries, as major bulk commodity exporters, Zhang noted.
"Such effects came in as a demonstration of the new development pattern, or 'dual circulation', proposed in last week's meeting of the Political Bureau of the Communist Party of China Central Committee, which underscores the domestic market as the mainstay while domestic and foreign markets can boost each other," said Zhang.
Shanghai increased airport screening on Saturday as imported coronavirus infections from countries such as Italy and Iran emerge as the biggest source of new cases in China outside Hubei, the province where the outbreak originated.Mainland China had 99 new confirmed cases on Friday, according to official data. Of the 25 that were outside Hubei, 24 came from outside China.To get more latest Shanghai news, you can visit shine news official website.
Shanghai, which had three new cases that originated from abroad on Friday, said it would step up control measures at the border, which had become “the main battlefield”.
At a news conference, Shanghai Customs officials said they city would check all passengers from seriously affected countries for the virus, among other airport measures.Shanghai already requires passengers flying in from such countries, regardless of nationality, to be quarantined for 14 days. They will now be escorted home in vehicles provided by the government.
Tighter screening has greatly lengthened waiting times at Shanghai’s Pudong International Airport - some passengers say they have had to wait as long as seven hours.
The Shanghai government vowed on Saturday to severely punish passengers who concealed infections.
Beijing police said on Saturday they would work with other departments to prevent imported infections. They said some members of a Chinese family flying in from Italy on March 4 had failed to fill in health declarations accurately, and later tested positive for the virus.In addition to the growing risk of imported infections, China faces a challenge in trying to get migrant workers back to work by early April.
So far, 78 million migrant workers, or 60% of those who left for the Lunar New Year holiday in January, have returned to work.
Yang Wenzhuang of the National Health Commission (NHC) said that the “risk of contagion from increased population flows and gathering is increasing ... We must not relax or lower the bar for virus control”.But new cases in mainland China continued to decline, with just 99 new cases on Friday, the lowest number the NHC started publishing nationwide figures on Jan. 20, against 143 on Thursday.
Most of these cases, which include infections of Chinese nationals who caught the virus abroad, were in the northwesterly Gansu province, among quarantined passengers who flew into the provincial capital Lanzhou from Iran between March 2 and 5.